- Studies that are NOT FDA regulated will continue to be reviewed and conducted under the Pre-2018 (“Old”) Common Rule (regardless of funding or support.)
- You are not required to transition these studies to the 2018 Common Rule
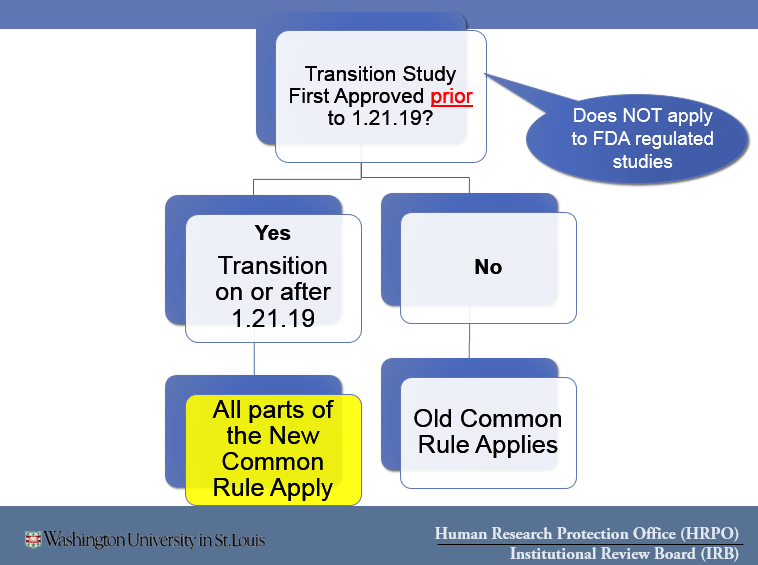
- You MAY transition your study to the 2018 Common Rule if that would be beneficial–see:Transitioning Studies First Approved BEFORE January 21, 2019 and are ONGOING.
- If you choose to transition your study to the 2018 Common Rule and it is NOT under FDA regulations the following changes will apply to your study
- New consent requirements including adding the Key Information section and other wording changes will be required
- Continuing Review will not be required if your study was originally approved by expedited review (minimal risk research) or if your study was originally reviewed by the full board as a more-than-minimal risk study but it has reached the point of data analysis or clinical follow-up only.
- The requirement to post a consent document to a federal public website will apply if your study meets the definition of a clinical trial
- If you choose to transition your study to the 2018 Common Rule and it is NOT under FDA regulations the following changes will apply to your study
- Studies that are FDA regulated and do NOT have any federal funding will continue to be reviewed and conducted under FDA regulations.
- The FDA will still require Continuing Review for all studies regardless of risk or status of the study
- No changes in how Modifications or REFs are reviewed
- The FDA does not require the Key Information section or other wording changes required in the 2018 Common Rule. However these changes do not conflict with any FDA regulations, so you may add the new consent items if you so choose.
- You will NOT be allowed to transition these studies to be reviewed under the 2018 Common Rule
- Studies that are BOTH FDA regulated and have federal funding or support will continue to be reviewed and conducted under both sets of regulations.
- The most restrictive rules apply where the two rules conflict.
- The FDA regulations will still require Continuing Review for all studies regardless of risk or status of the study
- No changes in how Modifications or REFs are reviewed
- You MAY transition your study to the 2018 Common Rule if that would be beneficial – see:Transitioning Studies First Approved BEFORE January 21, 2019 and are ONGOING.
- If you choose to transition your study to the 2018 Common Rule and it is also under FDA regulations
- The new consent requirements of the 2018 Common Rule including adding the Key Information section and other wording changes will be required
- FDA regulations will require that Continuing Review still be conducted
- The new 2018 Common Rule requirement to post a consent document used in a clinical trial will be required
- If you choose to transition your study to the 2018 Common Rule and it is also under FDA regulations
click diagram to enlarge
NOTE: “Equivalent Protections” in the diagram above indicates the IRB has some flexibility when a study is neither FDA regulated nor Federally funded or supported to apply flexibility in review if the protections put in place are equivalent to those required in the 2018 Common Rule and/or the FDA regulations.
Content current as of 10/5/2022