- Studies that are NOT FDA regulated will be reviewed and approved under the 2018 Common Rule (regardless of funding or support.)
- Studies that are FDA regulated and do NOT have any federal funding will be reviewed and approved under FDA regulations
- Studies that are BOTH FDA regulated and have federal funding or support will be reviewed and approved under both sets of regulations, with the most restrictive rules applied where the two rules conflict.
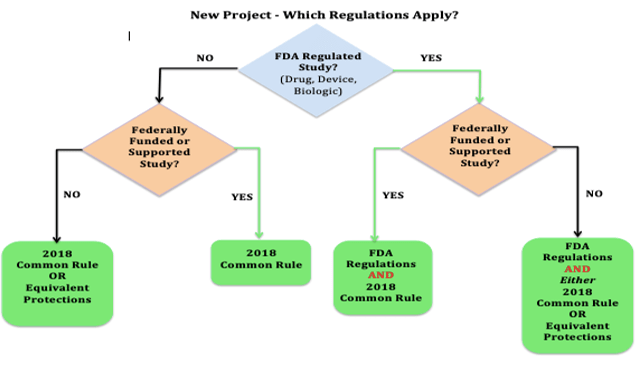
click diagram to enlarge
NOTE: “Equivalent Protections” in the diagram above indicates the IRB has some flexibility when a study is neither FDA regulated nor Federally funded or supported to apply flexibility in review if the protections put in place are equivalent to those required in the 2018 Common Rule and/or the FDA regulations.
Content current as of 10/5/2022