Terminology:
| Pre-2018 Common Rule | Federal Human Subjects Regulations in effect prior to January 21, 2019 |
| 2018 Common Rule | Federal Human Subjects Regulations in effect on or after January 21, 2019 |
________________________________________________________
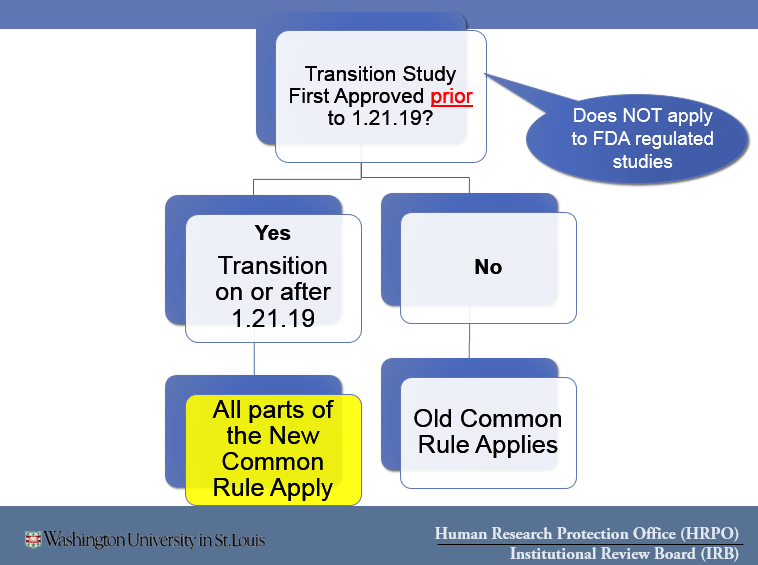
- Studies may only be transitioned to the 2018 Common Rule at the time of Continuing Review
- The IRB must conduct a complete expedited or full board review of your study under the 2018 Common Rule in order for it to transition.
- The IRB only has sufficient information at the time of Continuing Review to conduct this complete review.
- For those studies eligible to transition, a new question will appear as the first question on your Continuing Review application to ask if you want to transition.
- If you say YES to transitioning to the 2018 Common Rule, this is a permanent decision and the entire 2018 Common Rule applies to your study going forward until it closes.
- If you say NO to transitioning to the 2018 Common Rule, you will continue to be asked at each subsequent Continuing Review whether you want to transition at that time.
- You do NOT have to transition your study to the 2018 Common Rule. You may leave your study under the Pre-2018 Common Rule for the remaining life of the study.
- See OHRP Q&A for additional information on what new regulations apply if you choose to transition your study to the 2018 Common Rule.
- The IRB must conduct a complete expedited or full board review of your study under the 2018 Common Rule in order for it to transition.
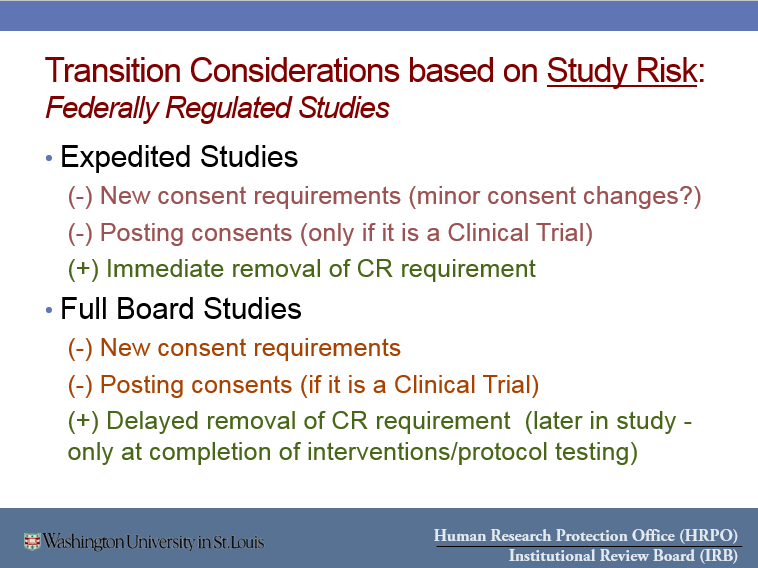
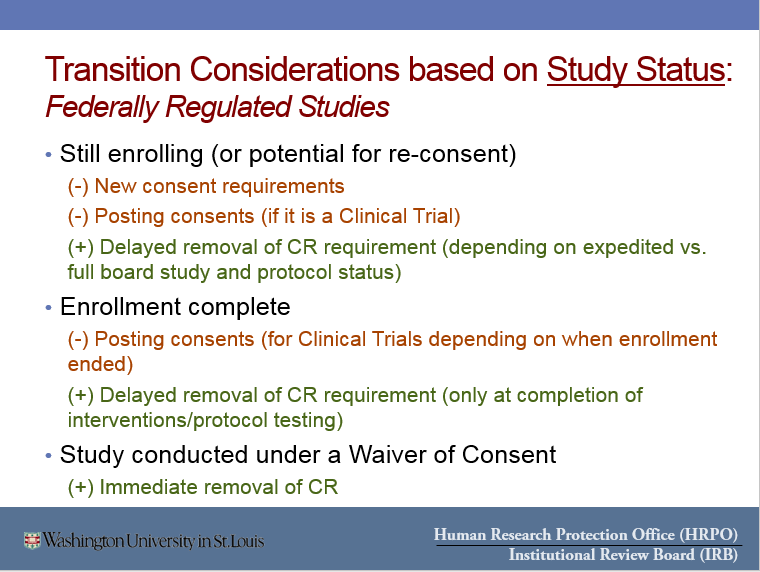
- Key reasons you might want to transition your study
Click image to enlarge

Click image to enlarge
Click image to enlarge
Click image to enlarge
Content current as of 10/5/2022